Many have oversimplified ideas about fertilizer as either good or bad for sustainability, soils, economy, or health. First, many factors and processes are important to understand, even if the terms are confusing and a bit complex.
Are “Chemical” fertilizers, “artificial” or “natural” nutrients in water-soluble forms?
Fertilizers, confusingly called “chemicals,” mostly consist of naturally occurring minerals and salts also found in healthy organic food and mineral food supplements, despite common beliefs. However, nitrate is not healthy to be consumed but is found in healthy green vegetables in small amounts. “Mineral” nitrogen fertilizer is today made synthetically, but they still consist of naturally occurring salts or molecules. Urea (= CO(NH2)2,) is called a mineral fertilizer but is actually an organic chemical also occurring in urine). Most fertilizers are water-soluble and, therefore, not widely found concentrated in nature, but large deposits exist of potassium chloride (KCl, sylvine, or sylvite), for example, exist. Like several fertilizer minerals, KCl deposits are not likely to be depleted for many centuries. Exchanging the relatively plant-toxic anion Cl- with a nutrient anion improves the fertilizer. It could be with nitrate (NO3-), phosphate e.g., HPO43-, or sulphate SO42-).
Often, they are processed to make them more soluble and concentrated (e.g., triple superphosphate, T.S.P.) and with more types of nutrients (e.g., Calcium Ammonium Phosphate, C.A.P.).
Water-soluble fertilizer can, like fresh organic matter, be risky to place too close and concentrated to seeds – particularly during drought. Some fertilizers contain less salt per kilo of nutrients (high concentration or ‘analysis’ fertilizers), and some are not acidifying soils. Strong acids are often used to make fertilizers soluble and enrich them with N, P, or sulphur (S) so unused residues can remain. However, fertilizer placement is sometimes an advantage, and at least P-fertilizers bind strongly to many clay soils and are best banded near seeds to be available and accessible.
Fertilizer challenges and solutions regarding acidification, micronutrient depletion, and heavy metals:
Mineral fertilizers are often blamed for acidifying soils (reducing pH). However, some nitrogen fertilizers (urea, and even more ammonium, NH4+, fertilizer) tend to do that without liming in humid and subhumid areas. However, ammonia (NH3) and Nitrate (NO3-) increase pH, and Calcium Ammonium Nitrate (C.A.N.) is largely having a neutral effect. The Calcium content is small but minimizes explosion risks. Ammonium is a form used more directly by plants than nitrate needing less energy, time, and micronutrients to use. It is not so easily washed out (leached) except in some acid tropical soils (variable charge soils). Ammonia liquid under pressure can be injected into the soil in bands that kills the directly affected organisms, but soon after nourishing increases biological activity. Liquid livestock manure and urine also contain much ammonia.
Depletion and removal of nutrient cations (called “bases” like K+, Ca2+, and Mg2+) can also acidify soils. It can happen with washing (leaching) down by infiltrating water, and plants are harvested without enough recycling of residues and no liming. Nitrogen fixation without recycling can also acidify. Acid cations are e.g., H+, Al3+ and similar cations forming part of the exchangeable acidity.
Root-soil reactions should be considered too for impact on pH, and it depends on fertilizer types applied.
Heavy metal content (mainly Cadmium (Cd)) can be a problem in some phosphate fertilizers – but processing rock phosphate may reduce the problem. Some organic manures can be high in Copper and used as fungicides even in organic farms for hoofs, fruit trees, coffee, etc. It is also permitted in organic farming as an important “natural” substance.
Depletion of other nutrients
Only applying a few nutrients, like N, N.P., or N.P.K., can, in some cases, give a shortage of others for crops, livestock, and even consumers. Fertilizers can supply any nutrients, but fertilizers with many nutrients can be more costly. Sometimes nutrient conservation, availability, uptake, and digestibility can be promoted in other ways – particularly for micronutrients. Calcium (Ca) and Sulphate (S) are beneficial parts of some fertilizers mainly used as N or P sources. These fertilizers include Calcium Ammonium Nitrate (C.A.N.), Ammonium Sulphate (A.S.), and the Ca and S containing Single Super Phosphate (S.S.P.) with Ca and S.
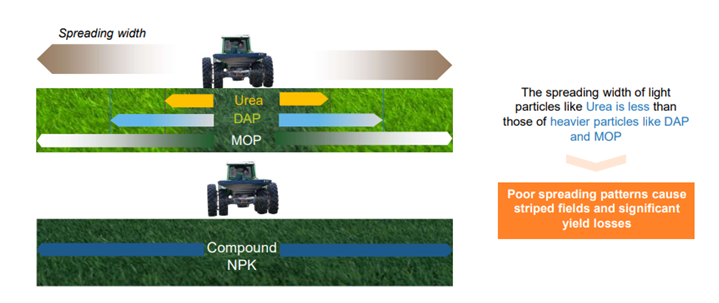
Figure 1. A uniform compound fertilizer is placed near a young tree. Mixing fertilizer into soil reduces nutrient loss. Knowledge of fertilizers is important for using them more sustainably, effectively, and safely.
Fertilizer forms
Fertilizers can be made in many forms to improve timely release and uptake by plants (or to be sold at a high price):
- The form of the fertilizer matters (e.g., particle size, coatings, combinations), as well as the time of application, spreading/placement, mixing with soil, etc. Big' super granulates' of urea are often used in wetland rice. New promising methods are being developed for foliar applications where absorption via soil and leaves can be challenging – particularly from the soil. Calcium Ammonium Nitrate is easier to spread uniformly than urea. Likewise, compound fertilizers spread better than simple mixtures of fertilizer aggregates. See the cover picture and Figure 1.
- Some nutrients are suited for foliar application, and some are not. P often scorches leaves. Ca, B, and Mn cannot move from treated leaves to other plant parts that may need the nutrient. This ability is poor for S, Cu, Fe, Zn, and Mo, and repeated spraying is needed. Nanotechnology may help for foliar applications, but many challenges remain, and it is often promoted without research comparing it well to other fertilizers used on plants. [1]
- Likewise, undocumented "boosters" and "stimulants" are sold – sometimes as "organic" and may be sold in small packages for a whole field but at best suited for micronutrients. Relevant innovations may occur too.
- Fully water-soluble fertilizers are released fast, but for analysis, slow-release fertilizers like rock phosphate or wood ash should also be extracted and for measurement with neutral ammonium citrate extraction [2]
- Fertilizers can contain one, two, or many nutrients and have various effects on soil pH and with concentrated placements. of soluble salts near seeds.
- In short, the right choice of fertilizer source, doses, timing, and place (application methods) (4 Rights, "4R") can help much with environmental, climate, and economic challenges regarding fertilizers.
- The space is insufficient for many important details. Free, updated online handbooks on more cost-effective and sustainable fertilizer use are available from the fertilizer industry (e.g., on sustainability and markets)[3] and by the International Fertilizer Association (more practical). [4]
Mineral fertilizer, organic matter, and other soil fertility strategies
"Chemical fertilizers can improve possibilities for using and neglecting other important soil fertility aspects. Several aspects are important and often misunderstood:
- Combining mineral and organic fertilizers was the most effective. Many long-term experiments in Europe have shown that applying mineral fertilizers year results in more (!) soil organic matter (S.O.M.) and crop (and fodder/manure) yields that can build S.O.M. than simply leaving fertilizer out and mining (depleting) the soil. Phosphate fertilizer can also contribute to soil aggregation by forming compounds with Aluminium or Calcium, while ammonium can disperse soil aggregates (NH4+). See Haynes and Naidu (1998)[5].
- Likewise, fertile soils can be more valuable and attractive to conserve, e.g., by terracing.
- However, in practical farming systems, fertilizers are often (mis)used as substitutes for other soil fertility and conservation methods such as intercrops, fallow fields, and cover crops. Using fertilizer, weed control, and tillage can be more profitable to intensify, at least in the short run. Likewise, soil challenges may not be solved even if they could. Fertilizers usually give higher yields when used right; this means less area is needed for food production, and cultivating fragile soils, forests, and nature may be profitable.
- Mineral fertilizer application can increase the production of plant residues and fodder, resulting in more manure and nutrient-rich urine. However, fertilizer can also reduce farmers' need to transport and use organic manures effectively, safely, and sustainably.
- Fertilizers can speed up early soil-protecting plant cover but also make intensive weed control more profitable by exposing soil.
- Nutrients like Nitrogen, Phosphorus, and Sulphur are needed for all organisms to build stable soil organic matter with carbon. Still, in high amounts, it can sometimes help bacteria break it down.
- Organic manure and residues vary in composition, and their nutrients are not always released when the plants can take them up, -and some coarse material may rather bind nutrients. Fallows and livestock manure are harder than fertilizer to use without polluting losses to water and air if high amounts are available. Advanced organic farms, e.g., in Denmark and Kenya, often buy nutrients as fodder from conventional farmers and maintain yield levels a bit below the Sustainable soil fertility includes many possibilities and challenges important to know for farmers. However, e.g., clear organic certifications and standards can still attract some consumers and inspire diverse and sustainable practices.
- Young trees can often benefit from some fertilizers, and the benefit may last long, but often it is not needed for survival and slow growth. Slow release of nutrients can be better in competition with other plants. Tree fallows followed by slash-and-burn cultivation is a traditional soil rejuvenation method, but too little land and time are available today to make it sustainable in most places in the traditional form. Improved low-cost tree establishment is needed for alternatives.
Recycling from burning and ash
When burning biomass (such as crop residues or wood) completely, all the organic matter and Nitrogen (N) are lost to the air -- and so are about half of the sulphur (S). Burning makes organic materials into minerals, some char, perhaps biochar, will usually be included and function as stable organic matter. Often, the effect of the nutrient-rich and alkaline ash is misleadingly attributed to the "biochar" in acid soil. It can store carbon, but the energy from incomplete burning may replace less fossil fuel than complete burning.
In very hot fires (e.g., above 800oC and fast airflow to dry material), much of potassium (K) is lost to the air, and K can, like Sodium and Silicate, form scaly, hard deposits on fans and heat exchangers in biomass burners. Likewise, ash particles, including Phosphate (P), can melt (=sinter) and form glassy gravel that only very slowly become available to plants. Also, highly alkaline and corrosive oxides like CaO are formed instead of moderately alkaline carbonates like CaCO3.
Such very hot fires can also cause higher energy loss from radiation in power stations and biomass burners on commercial farms. In contrast, by burning relatively moist biomass in moderate temperatures, moderate fire temperatures can use more steam for heat energy transfer (higher heat capacity). Reducing fire temperature sometimes causes incomplete polluting burning, e.g., in simple burners or when access to oxygen is restricted.
Ash is from burning field residues, trees or biomass in homes is often poorly distributed, and it can easily be eroded by wind or rain. The amount of fertilizer that ash can contribute may be small for an entire field. However, on small farms, it can be used to test if more nutrients than N or lime are needed by the plants or help legumes to start and for, N-fixing root nodules (nodulated) at infertile parts of the field.
Ash can also be used as an alkaline replacement for ammonia to make straw more digestible as fodder, but the Nitrogen from ammonia is used too. Ash can also be used to lime and fertilise legumes e.g. for protein-rich fodder.
Over time, removal of high or moderate nutrient yields from the farm requires a supply of nutrients from outside the farm in the form of manufactured fertilizer or other sources (e.g. bought fodder, manure and, in some cases, crushed rock phosphate). Release from soil minerals is not enough (except e.g. iron and other abundant micronutrients). It is important to use the right sources, doses, timing, and placements for crop needs. [6]
References:
[1] Husted S et al. (2022): What is missing to advance foliar fertilization using nanotechnology? In: Trends in Plant Science. 16 pp (in print). DOI: 10.1016/j.tplants.2022.08.017
[2] Phosphorus (Available) in Fertilizers – Direct Extraction Method, Official Methods of Analysis, 21st Ed. (2019) AOAC INTERNATIONAL, Gaithersburg, MD, Method 993,31
[3] Fertilizer Industry Handbook 2022 (yara.com)
[4] Drechsel, P, Heffer, P, Magen, H, Mikkelsen, R, Wichelns, D (2015): Managing Water and Fertilizer for Sustainable Agricultural Intensification. IFA, IWMI, IPNI and IPI, first edition, Paris, France, January 2015. And other books at : Library (fertilizer.org)
[5] Haynes, R, Naidu, R (1988): Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: a review. Nutrient Cycling in Agroecosystems 51, 123–137. https://doi.org/10.1023/A:1009738307837
[6] Nutrient Management Handbook IFA, WFO and GACSA 2016.
Sustainable Plant Nutrient Management (SPNM): An overview
Sustainable Nutrient management: Introduction to concept, strategies, and principles
Nutrient conservation and cycling
Mineral fertilizers (including ash) and sustainability
Biological Nitrogen Fixation and seeding Legumes for Soil Fertility
The importance and management of Phosphorus (P) and Potassium (K) in plant production
Ion charges and secondary (=meso) nutrients: Calcium, Magnesium and Sulphur
How important are the Micronutrients for plants
Soil and plant analysis and field observations
When are approaches to Plant Nutrient Management actually Sustainable?







