How nanotechnology is used in Agriculture
Nanotechnology in agriculture: The future is now!
Key Takeaways
- It is vital to meet the nutritional needs of the world’s population, which is growing rapidly. Nearly one-third of crops grown conventionally suffer damage, mostly due to pest infestation, microbiological assaults, natural disasters, poor soil quality, and a lack of available nutrients. To solve these problems, we urgently need more innovative technological advancements. On top of that, the inefficiency of agrochemicals and ecological concerns have diverted attention to finding a sustainable alternative to modern agriculture under the umbrella of nanobiotechnology (nano-agritech). In this regard, nanotechnology has contributed to the agrotechnological revolution with the potential to reform the resilient agricultural system while promising food security.
- During the last years, the agriculture industry has seen a significant increase in the market for nanotechnology with North America holding the largest market share for this sector worldwide, while Asia Pacific is anticipated to develop at the highest Compound Annual Growth Rate (CAGR) during the forecast period 2023-2030 according to advanced market research reports.
- Because the topic is really broad, in this article we will focus on the interaction of nanoparticles – NPs with plants, by studying how plants take in and absorb NPs, how they migrate inside the plant, and how they interact with plant cells is crucial. A second article will address recent advances in agro-nanotechnology like nano pesticides, nano fertilizers, NP-based biosensors for pesticide residue detection or agricultural post-harvest waste management. The toxicological impact of NP risk and health hazards in agriculture applications will also be reviewed in a future text.

1. Introduction
Sustainable agricultural productivity and global food security are the two major challenges to feeding the ever-growing population, estimated to reach ~10 billion by 2050. The global food production has already tripled during the Green Revolution (1). However, it still requires a massive up-scaling to meet the needs of an additional ~3 billion population; for instance, the rise of ~1 billion tonnes/year in cereal production is required to be achieved by 2050 (2). Although this agricultural intensification brings major concerns regarding environmental health because the injudicious use of agrochemicals is a potential hazard to diverse ecosystems and functions. Only a small proportion (1–25 %) of pesticides reach to target organism, and the rest are released into the environment. Therefore, the delivery of introduced agents must be in a targeted and stimuli-responsive manner to reach the specific tissues and organisms for maximizing the efficiency of crop production. Similarly, high doses of chemical fertilizers negatively affect plant nutrient uptake and utilization and have reduced around half of nitrogen, sulfur, potassium and phosphorus uptake efficiency (3). The inefficiency of agrochemicals and ecological concerns have diverted attention to finding a sustainable alternative to modern agriculture under the umbrella of nanobiotechnology (nano-agritech). In this regard, nanotechnology has contributed to the agrotechnological revolution with the potential to reform the resilient agricultural system while promising food security. Hence, research on the implications of nanotechnology on the agriculture industry has emerged as a crucial component of sustainable development. Due to the rising population, increased demand for food products, and growing consumer awareness of food safety, the government is investing in and promoting the adoption of new agriculture technologies, which is expanding the industry.
Additionally, established agrochemical businesses are the main drivers of the global agricultural nanotechnology market since they are investigating the potential of nanotechnologies to achieve high efficiency and greater technology penetration into agricultural components used for plants. Technology-driven mid-sized businesses that develop soil-enhancement solutions that encourage uniform water distribution, storage, and therefore water conservation have introduced a variety of nano-products designed exclusively for the agricultural sector to the market. The agriculture industry has seen a significant increase in the nanotechnology market (Figure 1) and the commercial agrochemical industry has conducted research to determine the possible benefits of nanotechnology in agriculture.
Farmers do not, however, use nanotechnology in agriculture due to a lack of knowledge (4). Since several nations, including the United States and Canada, were early adopters of agriculture nanotechnologies, North America holds the largest market share for this sector worldwide. Smart agricultural practices are becoming more critical due to the population boom. In the year 2020, Europe also had a significant market share. High adoption rates for agricultural nanotechnology, increased startup activity in agriculture innovation, and crowdfunding for agricultural innovations are all factors in the expansion of European agricultural technology. During the projection period, Asia Pacific is anticipated to develop at the highest Compound Annual Growth Rate (CAGR) (Figure 2). Due to the increased demand for processed foods and advancements in the agriculture industry, the Asia-Pacific nanotechnology market is predicted to rise significantly in the upcoming years. The Asia-Pacific region’s agricultural nanotechnology market is expanding due to rising demand for agricultural technologies, disposable income, and increasing population (4).
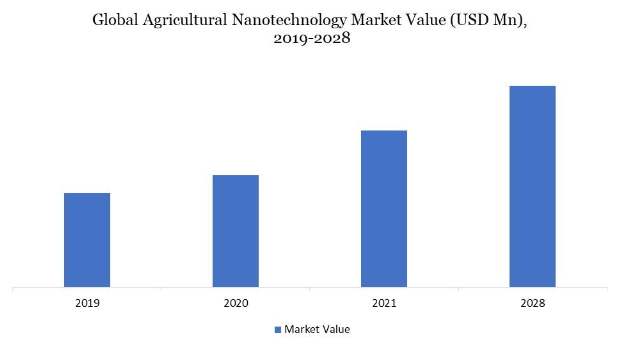
Figure 1. Global agricultural nanotechnology market value (USD Mn), 2019-2028 (4)
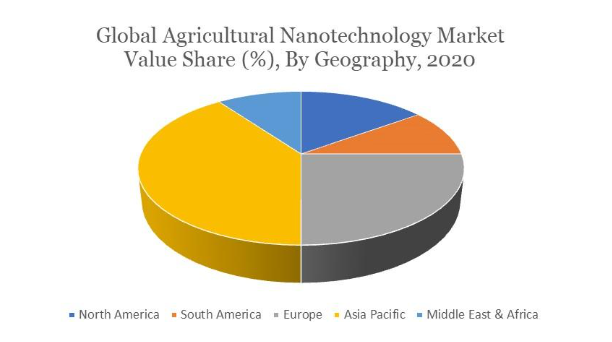
Figure 2. Global agricultural nanotechnology market value share (%), by geography, 2020 (4).
For all the above reasons, nanoparticles – NPs are becoming a new-age material to transform modern agricultural practices. Various nanoparticle-based formulations, including nano-sized pesticides, herbicides, fungicides, fertilizers, and sensors, have been widely investigated for plant health management and soil improvement. An in-depth understanding of plant and nanomaterial interactions opens new avenues toward improving crop practices through increased properties such as disease resistance, crop yield, and nutrient utilization. In addition, nanotechnology exploits the unique properties of NPs and develops nanosensors to detect pesticide residues and various soil parameters, such as pH and moisture. On top of that, it is vital to test the extent of toxicity of NP-based products before implementing them in the market. Nanotechnology-based products, such as smart agrochemical delivery systems using NPs as a carrier of active ingredients, are being continuously developed. The waste products of agriculture, such as soy hulls and wheat straws, can be converted into advanced bio nanocomposites with improved mechanical and physical properties for industrial purposes. Some progress is still needed to gain the practical advantages of nanotechnology, such as better design of processes, risk assessment of nano pesticides and nano fertilizers, and regulations (5).
2. NP interaction with plants
With the development of nanotechnology, nanoformulations for sustainable agriculture are regularly generated that are more effective and contain less contaminants (6). After entering the intricate plant-soil system, these new-age materials have the capacity to change the physiology of plants, which may be easily used to comprehend the after-effects (Figure 3).
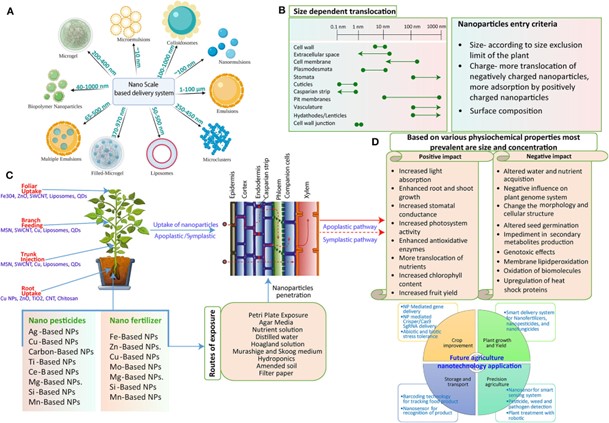
Figure 3. Potential interactions of NPs with the plant system are illustrated schematically. (A)
Several NPs are utilized as nano-carriers for carrying exogenous cargo. The size of these particles might range from 10 nm to more than 100 nm. (B) Interaction mechanism: The process by which NP interacts with and then distributes to the plants. Once NPs have successfully penetrated the outer layers, they travel along apoplastic and symplastic pathways to various plant cells and tissues. (C) The cell wall’s size exclusion limit (SEL) is 5–20 nm, the SEL of the Casparian strip is 1 nm, and the SEL of the plasmodesmata is 3–50 nm in diameter in plants. These exclusion limits place various restrictions on the mobility of NPs. The entry of bigger-sized NPs is made possible by the ability of some NPs to create larger pores in cell walls, plasmodesmata, and cuticles (lines with dotted corners depicted). Due to electrochemical interactions between them, NPs with various surface charges have varied absorption and translocation rates through the plant system. Additionally, the mobility is changed by the NPs’ surface alteration. (D) The numerous ways that plants react when exposed to NPs. NPs can have positive and negative effects on plants, mostly defined by their physiochemical characteristics. The most common characteristics are size and concentration, where higher concentrations have a negative effect and lower concentrations favor the plant system. On the other hand, size is crucial since small-size NPs can be easily absorbed by the plant, resulting in greater contact and a more powerful direct impact.
Furthermore, a lot of study and knowledge is required to achieve those objectives. Not only for creating and manufacturing nanocarriers and nanomaterials, but also for research into how such nanodevices interact with the environment and plant life. It is crucial to study how plants take in and absorb nanoparticles, how they migrate inside the plant, and how they interact with plant cells (6).
2.1 Plant absorption and uptake of nanoparticles (NPs)
The implementation and transformation of NPs are influenced by a number of factors, including plant, physiology, physicochemical properties of the NP itself, size, surface charge, shape, and potential interaction with plants and the environment (7). It is obvious that the characteristics of the nanoparticles will have a big impact on how they behave and if the plant can absorb them (7). As numerous barriers limited within the plants are in the range of micrometers (mm) to nanometers (nm), the size of the NPs must be taken into consideration as a critical parameter to study uptake in plants (5) (Figure 4). There are reports regarding the maximum dimensions plants allow for nanoparticles to travel and allocate inside the cells, often with a size exclusion limit of 40–50 nm (8), (9). For instance, the cells that make up the cuticle membrane are found in the foliar epidermis. The epidermis comprises stomata, which contain two guard cells and provide an opening for gaseous exchange that is between 3 to 12 µm wide and 10 to 30 µm long. NPs can therefore pass through the plants due to these stomatal holes. Additionally, the stomata trichrome and the epidermis’s cuticle layer have quite different permeability capabilities (Figure 3B). The cuticle layer, on the other hand, is more significantly present on the leaf epidermis and has a size exclusion limit in the range of nm (10), (11) (Figures 3B).
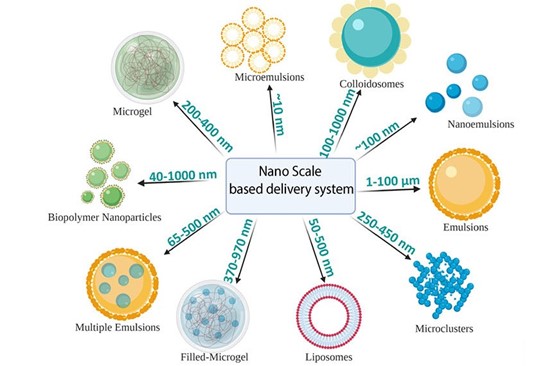
Figure 4. Schematic representation of nanoscale-based delivery systems and their sizes (5).
In addition to this, another element affecting uptake is the type of nanoparticle and its chemical composition (12), (13), whereas morphology has at times been shown to be a determining factor also (14). The properties of the nanomaterial’s absorption and accumulation by the plant can be significantly altered and changed by functionalizing and coating its surface (15).
2.2 Internal NP movement in plants
The apoplast and the symplast are two pathways for the nanoparticles to travel through tissues once they have entered the plant (7) (Figure 5C). Apoplastic movement occurs outside the plasma membrane through the extracellular spaces, cell walls of adjacent cells, and xylem vessels (16). In contrast, symplastic transport involves the flow of water and other substances through specialized structures called plasmodesmata and sieve plates between the cytoplasm of adjacent cells (17). The apoplastic pathway is crucial for radial movement inside plant tissues. It enables the transfer of nanomaterials to the vascular tissues and root central cylinder for further movement to the aerial part. After entering the central cylinder, nanoparticles can travel through the xylem and toward the aerial part by following the transpiration stream (18). However, entering the xylem via the root requires moving past a barrier to the apoplastic pathway, the Casparian strip, which must be carried out following a symplastic way (19) via endodermal cells (Figure 5C). The Casparian strip may block and accumulate some nanomaterials (18). Nanomaterials must cross the cuticle’s barrier in the case of foliar sprays by taking either the lipophilic or the hydrophilic route (19). It’s crucial to understand how nanomaterials move inside plants since it could indicate where they might end up and accumulate and which areas of the plant they can access.
For instance, if a certain type of nanoparticle is transported mostly by the xylem and not the phloem, it will likely flow primarily from the roots to the shoots and leaves rather than downwards. Therefore, it is best to apply the nanoparticle to the roots in order to get a good distribution in the plant. On the other hand, foliar spraying should be used for application if the nanoparticles exhibit good phloem translocation (application-related NP uptake). Another crucial factor to take into account when trying to prevent additional human or animal ingestion of nanomaterials is the risk that nanomaterials moving along the phloem would accumulate in plant organs functioning as sinks, such as fruits (21), grains (22), flowers (23) and young leaves (24).
However, translocation is not always limited to a single cell type, and nanomaterials may migrate laterally between the xylem and phloem (25).
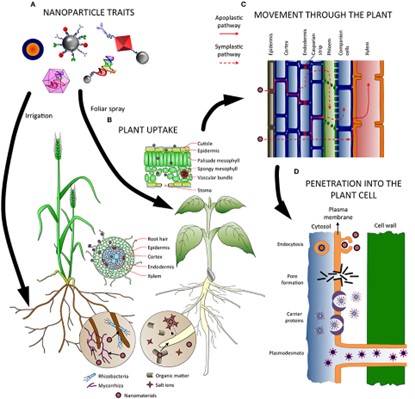
Figure 5. Factors influencing absorption, uptake, transport and penetration of NPs in plants. (A)
Nanoparticle characteristics influence both the application method and how they are absorbed and transported throughout the plant. (B) Nanoparticles in the soil may interact with substances and microbes, which may help or hinder their absorption. Depending on the entry site (roots or leaves), a number of tissues (epidermis, endodermis) and obstacles (Casparian strip, cuticle) must be passed before accessing the vascular tissues. (C) NMs can migrate up and down the plant via the apoplastic and/or symplastic pathways and switch between them using radial movement. (D) Several methods, including endocytosis, pore formation mediated by carrier proteins, and plasmodesmata, have been postulated for the internalization of nanoparticles inside the cells.
However, Zhu et al. (2012) discovered that the roots of radish and ryegrass accumulated greater amounts of gold nanoparticles than those of rice and pumpkin and that positively charged gold nanoparticles were absorbed by the roots more quickly than negatively charged ones, while the latter were more effectively transported to the aerial parts (surface charge-related NP uptake). This behavior was attributed to the presence of a negative charge in plant cell walls, which encouraged the accumulation of positively charged nanoparticles in tissues and hampered their movement within the plant (26).
2.3 Plant cell interaction with nanomaterials
Nanomaterials must be internalized by the plant cell and cross the plasma membrane to go through the symplastic route (Figure 5C). There are numerous methods through which nanoparticles can accomplish this (endocytosis, pore formation, carrier proteins, plasmodesmata and ion channels) (27, 28, 29, 30, 31, 32, 33) (Figure 5D). However, these pathways are better understood in animal cells and less well-known in plants. Another important question is how cells absorb nanoparticles, as this would again affect the practical use of nanomaterials. Endocytosis appears to be the best method for delivering chemicals into particular cell organelles. On the other hand, pore creation should be the most direct method for distribution in the cytosol. To treat crop systemic illnesses and infections, we may also be interested in nanomaterials that enter other species, such as bacteria or fungi, rather than the plant cell (34).
2.4 Techniques to study NP uptake, quantification, and translocation
Additionally, more crucial data is required to measure the uptake and translocation of NPs inside plants and as released in the environment. Potential methodologies must be developed to monitor the interaction between plants and nanomaterials – NMs.
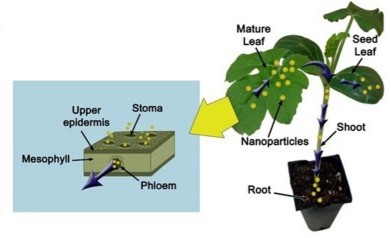
Figure 6. Illustration showing the movement of nanoparticles by phloem from the leaf to the root. Gas phase uptake of nanoparticles through stomata opening is shown in the inset.
Using a vibrating sample magnetometer, researchers were able to measure the assimilation and concentration of iron (Fe3O4) NPs in pumpkin (Cucurbita maxima) plants by confirming their presence in the plant’s roots and leaves (35). Likewise, by utilizing their magnetic properties, such as their temperature and magnetic field dependence on magnetization, Fe3O4 NPs can also be monitored and tracked in plant organs (36). The inability to distinguish between intact Fe3O4 NPs and leached ions is the main issue with tracking and translocating Fe3O4 NPs, which can also be resolved by combining different spectrometry and spectroscopy techniques (37). Since NMs can easily be transformed once they enter the plant, mass spectrometry-based analytical techniques aid in differentiating between their many chemical forms. For example, in order to evaluate the uptake mode and accumulation of gold nanoparticles (Au NPs) in watermelon plants (38) (Figure 6). These prospective strategies’ key benefit is their cumulative power or combination use. Although microscopy techniques have an obvious advantage in analysing NPs in various samples, they also have some significant drawbacks, such as sample preparation, a limited area of analysis, and limited 3-D imaging, necessitating alternative approaches.

3. Conclusion
The number of studies examining the relationship between NMs and plants has significantly expanded during the past few years. Though the full picture is incredibly difficult to grasp, we are starting to see some perspectives on how it operates. There are certain issues when we try to put this knowledge into practice because Agricultural Science draws on knowledge already tried and tested in Pharmacology and Medicine.
First, whereas agriculture deals with more than 7,000 cultivated plant species, medicine concentrates on one species, the human (or two or three if we include animals for preliminary tests and trials) (39) because plant physiology influences how nanoparticles interact with plants, so this condition makes it difficult to generalize research findings from one crop to another.
Secondly, the cost of developing nanomaterial production may make it difficult to use them for application in the fields. The use of nanoparticles as nanocarriers in plants resulted in a number of positive results. However, due to the high cost (both in terms of time and money) of generating large quantities of the nanomaterial for greenhouse or field experiments, some studies are only carried out with a few plants or organisms in vitro or in a growth chamber. While a (relatively) high manufacturing cost can be tolerated for medicinal applications, this is not feasible for agricultural production, and nanomaterials must be manufactured in large quantities at extremely low costs. Promising research in this approach involves using natural polymers like chitosan (40). These polymers are simple to synthesize and are derived from naturally occurring substances, such as chitin from the exoskeleton of crustaceans (for chitosan) and alginate from brown algae (41). They can be obtained in high amounts at a low cost.
Thirdly, this approach includes releasing nanoparticles inside crops and into fields, raising the first red flag: are these nanodevices safe for consumption by humans and animals? Although nanotoxicological research is being developed and performed in various ways, even involving the food chain (24). Chitosan and alginate, already applied in the food sector due to their lack of toxicity, biodegradability, and edibility, are the right materials for this purpose, as previously mentioned. A second article will address recent advances in agro-nanotechnology like nano pesticides, nano fertilizers, NP-based biosensors for pesticide residue detection, or agricultural post-harvest waste management. The toxicological impact of NP risk and health hazards in agriculture applications will also be reviewed in a future text.
Although agricultural uses of nanotechnology have good potential, there is still a long way to go before they can be implemented. Although it is impossible to predict every aspect of how a nanodevice will function in a specific crop, we must begin with actual field and in-plant tests to address some of the abovementioned issues (namely increasing production and avoiding dangerous and toxic compounds).
You can read
How nanotechnology is used in Food Industry
References:
- World Health Organization., 2019
- De Vries, W., Kros, J., Voogd, J.C., Ros, G.H. (2023) Integrated assessment of agricultural practices on large scale losses of ammonia, greenhouse gases, nutrients and heavy metals to air and water. Sci. Total Environ. 857, 159220. https://doi.org/10.1016/jscitotenv.2022.159220
- Sachdeva, S., Kumar, R., Sahoo, P.K., Nadda, A.K. (2023) Recent advances in biochar amendments for immobilization of heavy metals in an agricultural ecosystem: a systematic review. Environ. Pollut. 120937. https://doi.org/10.1016/jenvpol.2022.120937
- https://www.datamintelligence.com/research-report/agricultural-nanotechnology-market
- Mittal, D., Kaur, G., Singh, P, Yadav, K. and Ali, S.A. (2020) Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2:579954. https://doi.org/10.3389/fnano.2020.579954
- Fraceto, L. F., Grillo, R., de Medeiros, G. A., Scognamiglio, V., Rea, G., and Bartolucci, C. (2016). Nanotechnology in agriculture: which innovation potential does it have? Front. Environ. Sci. 4:20. https://doi.org/10.3389/fenvs.2016.00020
- Pérez-de-Luque, A. (2017). Interaction of nanomaterials with plants: what do we need for real applications in agriculture. Front. Environ. Sci. 5:12. https://doi.org/10.3389/fenvs.2017.00012
- Sabo-Attwood, T., Unrine, J. M., Stone, J. W., Murphy, C. J., Ghoshroy, S., Blom, D., et al. (2012). Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 6, 353–360. https://doi.org/10.3109/17435390.2011.579631
- Taylor, A. F., Rylott, E. L., Anderson, C.W., and Bruce, N. C. (2014). Investigating the toxicity, uptake, nanoparticle formation and genetic response of plants to gold. PLoS ONE 9, 93793. https://doi.org/10.1371/journal.pone.0093793
- Wang, P., Lombi, E., Zhao, F. J., and Kopittke, P. M. (2016). Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 21, 699–712. https://doi.org/10.1016/j.tplants.2016.04.005
- Wang, X., Yang, X., Chen, S., Li, Q., Wang, W., Hou, C., et al. (2016). Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant. Sci. 6:1243. https://doi.org/10.3389/fpls.2015.01243
- Ma, X., Geisler-Lee, J., Deng, Y., and Kolmakov, A. (2010). Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci. Total Environ. 408, 3053–3061. https://doi.org/10.1016/j.scitotenv.2010.03.031
- Rico, C. M., Majumdar, S., Duarte-Gardea, M., Peralta-Videa, J. R., and Gardea-Torresdey, J. L. (2011). Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 59, 3485–3498. https://doi.org/10.1021/jf104517j
- Raliya, R., Franke, C., Chavalmane, S., Nair, R., Reed, N., and Biswas, P. (2016). Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 7:1288. https://doi.org/10.3389/fpls.2016.01288
- Judy, J. D., Unrine, J. M., Rao, W., Wirick, S., and Bertsch, P. M. (2012). Bioavailability of gold nanomaterials to plants: importance of particle size and surface coating. Environ. Sci. Technol. 46, 8467–8474. https://doi.org/10.1021/es3019397
- Sattelmacher, B. (2001). The apoplast and its significance for plant mineral nutrition. New Phytol. 149, 167–192. https://doi.org/10.1046/j.1469-8137.2001.00034.x
- Roberts, A. G., and Oparka, K. J. (2003). Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 26, 103–124. https://doi.org/10.1046/j.1365-3040.2003.00950.x
- Sun, D., Hussain, H. I., Yi, Z., Siegele, R., Cresswell, T., Kong, L., et al. (2014). Uptake and cellular distribution, in four plant species, of fluorescently labeled mesoporous silica nanoparticles. Plant Cell Rep. 33, 1389–1402. https://doi.org/10.1007/s00299-014-1624-5
- Robards, A. W., and Robb, M. E. (1972). Uptake and binding of uranyl ions by barley roots. Science 178, 980–982. https://doi.org/10.1126/science.178.4064.980
- Schönherr, J. (2002). A mechanistic analysis of penetration of glyphosate salts across astomatous cuticular membranes. Pest Manag. Sci. 58, 343–351. https://doi.org/10.1002/ps.462
- Servin, A.D.,Morales,M. I., Castillo-Michel, H., Hernandez-Viezcas, J. A.,Munoz, B., Zhao, L., et al. (2013). Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ. Sci. Technol. 47, 11592–11598. https://doi.org/10.1021/es403368j
- Lin, S., Reppert, J., Hu, Q., Hudson, J. S., Reid,M. L., Ratnikova, T. A., et al. (2009). Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5, 1128–1132. https://doi.org/10.1002/smll.200801556
- Cifuentes, Z., Custardoy, L., de la Fuente, J. M., Marquina, C., Ibarra, M. R., Rubiales, D., et al. (2010). Absorption and translocation to the aerial part of magnetic carbon-coated nanoparticles through the root of different crop plants. J. Nanobiotechnology 8:26. https://doi.org/10.1186/1477-3155-8-26
- Koo, Y., Wang, J., Zhang, Q., Zhu, H., Chehab, E. W., Colvin, V. L., et al. (2015). Fluorescence reports intact quantum dot uptake into roots and translocation to leaves of Arabidopsis thaliana and subsequent ingestion by insect herbivores. Environ. Sci. Technol. 49, 626–632. https://doi.org/10.1021/es5050562
- Pate, J. S. (1975). “Exchange of solutes between phloem and xylem and circulation in the whole plant,” in Transport in Plants I, eds M. H. Zimmermann and J. A. Milburn (Berlin; Heidelberg: Springer), 451–473.
- Zhu, Z. J., Wang, H., Yan, B., Zheng, H., Jiang, Y., Miranda, O. R., et al. (2012). Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Environ. Sci. Technol. 46, 12391–12398. https://doi.org/10.1021/es301977w
- Etxeberria, E., Gonzalez, P., Baroja-Fernandez, E., and Romero, J. P. (2006). Fluid phase endocytic uptake of artificial nano-spheres and fluorescent quantum dots by sycamore cultured cells: evidence for the distribution of solutes to different intracellular compartments. Plant Signal. Behav. 1, 196–200. https://doi.org/10.4161/psb.1.4.3142
- Serag, M. F., Kaji, N., Gaillard, C., Okamoto, Y., Terasaka, K., Jabasini, M., et al. (2011). Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 5, 493–499. https://doi.org/10.1021/nn102344t
- Nel, A. E., Mädler, L., Velegol, D., Xia, T., Hoek, E. M., Somasundaran, P., et al. (2009). Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557. https://doi.org/10.1038/nmat2442
- Rico, C. M., Majumdar, S., Duarte-Gardea, M., Peralta-Videa, J. R., and Gardea-Torresdey, J. L. (2011). Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 59, 3485–3498. https://doi.org/10.1021/jf104517j
- Schwab, F., Zhai, G., Kern, M., Turner, A., Schnoor, J. L., and Wiesner, M. R. (2015). Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants – Critical review. Nanotoxicology 10, 257–278. https://doi.org/10.3109/17435390.2015.1048326
- Roberts, A. G., and Oparka, K. J. (2003). Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 26, 103–124. https://doi.org/10.1046/j.1365-3040.2003.00950.x
- Zhai, G., Walters, K. S., Peate, D. W., Alvarez, P. J., and Schnoor, J. L. (2014). Transport of gold nanoparticles through plasmodesmata and precipitation of gold ions in woody poplar. Environ. Sci. Technol. Lett. 1, 146–151. https://doi.org/10.1021/ez400202b
- Rispail, N., De Matteis, L., Santos, R., Miguel, A. S., Custardoy, L., Testillano, P., et al. (2014). Quantum dots and superparamagnetic nanoparticles interaction with pathogenic fungi: internalization and toxicity profile. ACS Appl. Mater. Interfaces 6, 9100–9110. https://doi.org/10.1021/am501029g
- Zhu, H., Han, J., Xiao, J. Q., and Jin, Y. (2008). Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 10, 713–717. https://doi.org/10.1039/b805998e
- Govea-Alcaide, E.,Masunaga, S. H., De Souza, A., Fajardo-Rosabal, L., Effenberger, F. B., Rossi, L. M., et al. (2016). Tracking iron oxide nanoparticles in plant organs using magnetic measurements. J. Nano. Res. 18:305. https://doi.org/10.1007/s11051-016-3610-z
- Ju, M., Navarreto-Lugo,M., Wickramasinghe, S., Milbrandt, N. B., McWhorter, A., and Samia, A. C. (2019). Exploring the chelation-based plant strategy for iron oxide nanoparticle uptake in garden cress (Lepidium sativum) using magnetic particle spectrometry. Nanoscale 11, 18582–18594. . https://doi.org/10.1039/C9NR05477D
- Raliya, R., Franke, C., Chavalmane, S., Nair, R., Reed, N., and Biswas, P. (2016). Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci. 7:1288. https://doi.org/10.3389/fpls.2016.01288
- Khoshbakht, K., and Hammer, K. (2008). How many plant species are cultivated? Genet. Resour. Crop Evol. 55, 925–928. https://doi.org/10.1007/s10722-008-9368-0
- Maruyama, C. R.,Guilger,M., Pascoli,M., Bileshy-José,N., Abhilash, P. C., Fraceto, L. F., et al. (2016). Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 6:19768. https://doi.org/10.1038/srep19768
- Campos, E. V., de Oliveira, J. L., Fraceto, L. F., and Singh, B. (2015b). Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 35, 47–66. https://doi.org/10.1007/s13593-014-0263-0
- Azevedo, M. A., Bourbon, A. I., Vicente, A. A., and Cerqueira, M. A. (2014). Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 71, 141–146. https://doi.org/10.1016/j.ijbiomac.2014.05.036










































































