Nitrogen (N): Essential for plant growth and yield but can have high costs to farmers, the environment, and health
Nitrogen (N): Forms, doses, and effects
Nitrogen is very important to know well because it is:
- the nutrient most often deficient and needed in the highest quantity,
- the most costly to supply as fertilizer,
- the easiest lost to the environment,
- the most likely to be important for water pollution (if the water plants have enough phosphorus),
- the nutrient that can threaten light-demanding rare plants from air pollution,
- the most energy-demanding to produce, the only real synthetic fertilizer nutrient,
- the forms found in fertilizers are also used by plants from manure, important for the quality of crops (both positive and negative),
- only one that can be added to a farm in big amounts without getting nutrients from other areas but the air,
- found in several forms in nature that differ much, and,
- important for all other nutrients and building soil organic matter, which both need enough plant growth and a ratio of Carbon (C) and N of about 30:1; else, plant residues may build up and burn and not be used for fodder either.
Nitrogen (N) forms
As indicated above, Nitrogen occurs in several forms, and N can easily be lost. E.g., Ammonia (NH3) is released when organic matter like manure decay. With access to oxygen, it usually gets oxidized (nitrification) to nitrate (NO3–) that may be leached down, taken up by plants, or denitrified to other forms lost to the air. See Cover picture (Nitrogen (N) deficiency causes yellowing first of the lower leaves. It can be uniform or in a V-shape from the tip. With permission: Crop Scout Pocket Guide for Maize, African Plant Nutrition Institute (APNI) 2021.)
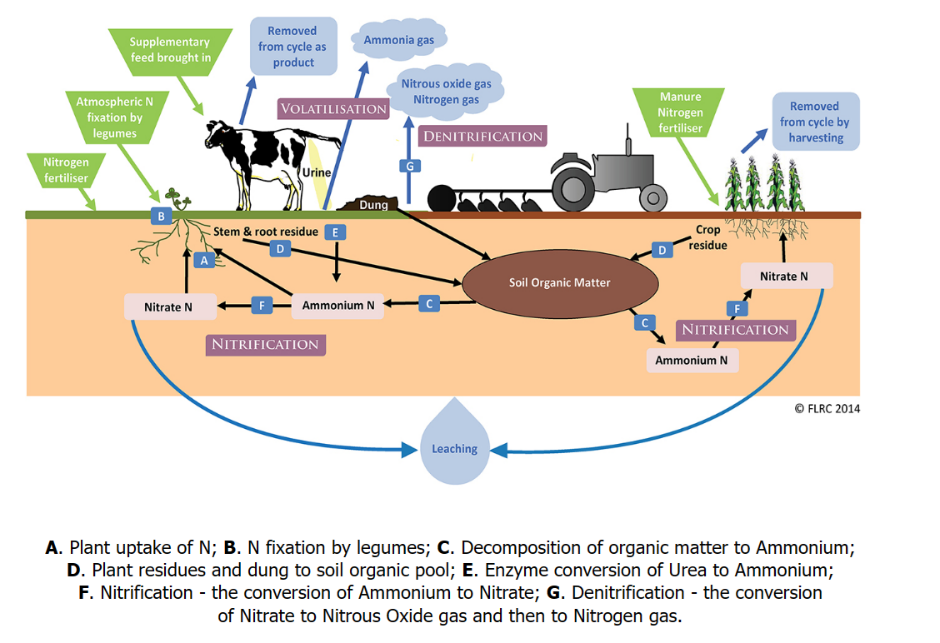
Figure 1. Nitrogen forms and the major flows, pools and transformations in agriculture. Permission: Farmed Landscape Research Centre, FLRC, New Zeeland.
In addition to what is mentioned in the cover figure: -loss or misplacement from erosion of fertilizer and organic matter can occur too, -a part of harvested nutrients can be recycled, and small supplies of N can come from free-living N-fixing organisms and ammonia-polluted air. The greenhouse gas N2O can also be lost to air from temporarily wet N-rich sites.
460 L ammonia (NH3) gas can be dissolved in 1 L of water. Ammonia can also be lost from solutions as gas from hot, windy, non-acidic surfaces. Organic acids from fermented organic matter will also oxidize if they have access to air, and neither compost nor slurry is acidic.
Applying wood ash to compost will increase pH, and if it is at the surface, as in many African households, it can be important. However, the pH of compost is not easily changed because it is strongly buffered against change.
Ammonium is released from organic matter and ammonium fertilizers. Acids supply protons (H+) and can change ammonia (NH3) to the positive ion (=cation) ammonium (NH4+). Ammonium fertilizers without calcium and nitrate do acidify soils without liming.
In aerated soils, most ammonium changes to nitrate (NO3–), which is easily washed out of the root zone (leached) in most soils. However, some old tropical soils are good at retaining nitrate when they are acid (high H+ levels) because they then get a positive charge. They are named variable charge soils.
Reducing N-leaching is essential, e.g., by delayed and divided doses (over 2-3 split doses per season). The cost of N should be considered (target high economic yield, not maximum yield) as well as timing with crop demand, water flows, nitrate formation (temperature and applied inhibitors), and available residues. N.P. or N.P.K. fertilizer is often applied at seeding time or 2 weeks after, and an 18:20 N:P fertilizer when, e.g., maize is knee high. Di-Ammonium Phosphate (D.A.P.) is an N.P. fertilizer with an N:P concentration per 100 kg.
Good timing of organic manure applications is more complex. For rapidly available green manure from one N-fixing tree legume, applying two weeks after seeding maize was significantly better than at seeding time when using a single application. It was the tropical Gliricidia sepium that had a low level of protein-binding tannins (polyphenols) and fibres in the leaves. Similar results have been found for Leucaena tree foliage having similar content. The application at seeding time was better for the moderately tannin-rich Calliandra calothyrsus tree legume. Tannins can be field-tested simply by “tasting” if they make the tongue stick a bit to the mouth. The high content of fibres (or lignin) can also delay release–field tests by crushing the leaves by hand. Green plant parts tend to be high in N. Seeds may also be a source of N at the start of the season. So can soil aggregates that are broken up in, e.g., a dry season period, so microbes get access to digest more organic matter when the rain starts again (the Birch effect)[1]. See also the article on nitrogen-fixing plants and their establishment by Mandal (2023) at Wikifarmer Library.
To reduce vaporization (loss to the air) from urea (CO(NH2)2), ammonia, N-rich organic manures, and particularly slurry, early incorporation is important and a law requirement within 24 hours in some countries.
The possible or actual nitrogen concentration in the organic matter varies much between sources, methods and countries. Likewise, N-containing fertilizers vary greatly in N concentration, see Figure 2.
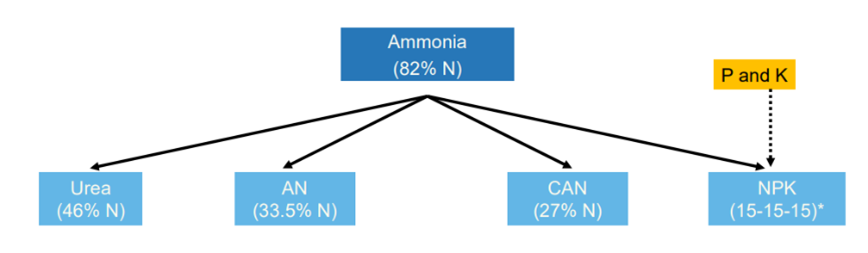
Figure 2. Theoretically possible Nitrogen (N) concentration varies much in N fertilizers. Ammonium Nitrate (AN) is sold has as safer mixes; Calcium Ammonium Nitrate (CAN) or various NPKs e.g. 15% N. Ammonium Sulphate: 21%. Yara Fertilizer Yearbook
Reducing Nitrogen pollution while optimizing profits rather than yields
For example, Denmark reduced (but still substantial) use of N-fertilizers has also been combined with higher yields and less pollution via research, breeding, — and regulations, including mandatory use of catch-crops and other ways to keep fields with plants almost all the year. Organic manures are the most difficult to fit precisely with the timing and dose of crop needs if used alone. Limits for maximum doses of both fertilizer and manures are imposed. More fertilizer means more feed and manure and less need for the farmer to transport and use the manure optimally. While each extra kilo of Nitrogen can give around 10 kg more yield, higher amounts will not help much and may not be profitable. Accordingly, farmers will get more profits and fewer pollutions when they do not apply N to get the highest yields, but to the point when an extra kilo of N has the same value on the farm as the extra (marginal) increase in yields. This is a general principle of maximizing profits based on a diminishing return to the scale of inputs. For other nutrients lasting longer in the soils, maintaining an adequate level by compensating for removal. A challenge is to predict how much N the crop should be supplied as it depends on variations in the field, rainfall, amounts, and availability of other sources of N (and of other nutrients), plant establishment, and the actual water availability in the season. The better supply of other growth factors, the more N can be taken up, so adjusting (or omitting) a top dressing is essential.
Thus, it is important to know that the cause(s) of variations of growth in the field is caused by a lack of N or other growth factors (e.g., too dry or wet soil or P deficiency).
Without variation, the yield response curve to the limiting growth factor may be constant and linear until another limitation stops it. Too high doses risk reducing yields and quality.
N -supply should be timed well with its release (e.g., from organic matter), plant uptake, and risk of loss by leaching or to air by volatilization as N2 or the greenhouse gas nitrous oxides (N2O). Well-timed split doses of N-fertilizers can reduce loss and slow the release of main nitrate (NO3–) like big super granules used in wet rice (paddy).
High N-doses can cause falling (lodging) and too much nitrate in water and green crops
Too high N-doses can be a problem. Even modern varieties can grow too tall and heavy and fall when they mature on a windy day.
Groundwater can be polluted with, e.g., nitrate (NO3–) levels above 10-50 parts per million (ppm). Nitrate levels can get too high in fodder, e.g., when N-rich manure or slurry is deposited in too high amounts to get rid of it in green vegetables. Nitrates can change into harmful forms that may cause cancer but eating fruits, vegetables, etc., with antioxidants can help as antioxidants. Babies can get bluish or purple fingertips (cyanotic) for this or other reasons and a combination of causes, e.g., in the densely populated, sandy Gaza strip. In extreme cases, a poor oxygen supply in infants can occur,[2] but e.g. in Denmark, such ‘blue babies’ have only been found when animal waste polluted a few old-fashion open wells before 1965, where polluted surface water in a few cases could run directly into them.
Impacts on pest, diseases, drought tolerance, and product quality
Too high N:K or N:P ratios can make plants more vulnerable to attack by pests and diseases when plants lack nutrients important for support structures.
A high N:P ratio can also delay maturity.
Any strong nutrient deficiency can reduce both top and root development. Moderate nutrient deficiencies, particularly for P, as the limiting factor for growth, can mean that roots can get nutrients to grow before they reach the top. Some trials and experts do not consider the severe deficiency.
High soluble salt concentration or problematic pH can also be a problem where fertilizers are placed near (or in contact) germinating crop seeds, particularly during droughts; mixing with soil or a 2-5 cm distance at least is needed. Fertilizers with many nutrients compared to other salts are called concentrated or high-analysis fertilizers.
While ammonium sulphate fertilizer is relatively cheap and acidifying, the sulphate may sometimes help and is like calcium tolerated better than sodium and chloride ions. Sulphur deficiencies can be mistaken for nitrogen deficiency, and it usually occurs after some years when only other nutrients have been applied.
Adequate or high supply of N can increase protein concentration, e.g., in wheat and baking quality, but it is mainly in the humid tropics (where wheat cannot grow and be stored well) that protein deficiency is widespread.
References:
[1] Les Henry (2021): The Birch effect on our soils in 2021 – Grainews. Les Henry: The Birch effect on our soils in 2021 – Grainews
[2] Ward M H et al. (2018) Drinking Water Nitrate and Human Health: An Updated Review – P.M.C. (nih.gov). International Journal of Environmental Research and Public Health 15, 1557. https://doi.org/10.3390/ijerph15071557. IJERPH | Free Full-Text | Drinking Water Nitrate and Human Health: An Updated Review (mdpi.com)
More reading:
FAO (2018): The 10 Elements of Agroecology. 10 elements | Agroecology Knowledge Hub | Food and Agriculture Organization of the United Nations (fao.org)
Fertilizer Industry Handbook 2022 (yara.com)
Mucheru-Muna M, Mugende D, Pypers P, Mugwe J, Kung’u J, Vanlauwe B, Merckx R (2014): Enhancing maize productivity and profitability using organic inputs and mineral fertilizer in central Kenya small-holder farms. Experimental Agriculture, 50(2), 250-269. doi:10.1017/S0014479713000525
Mudombi-Rusinamhodzi G and Rusinamhodzi L (2022): Food sovereignty in sub-Saharan Africa: Reality, relevance, and practicality. Front. Agron. 4:957011. doi: 10.3389/fagro.2022.957011.
Mandal (2023): Biological Nitrogen fixation and seeding legumes for soil fertility. Wikifarmer Library.
Mandal (2023): When is nutrient management sustainable -and how does it fit with sustainability approaches? Wikifarmer Library.
Nitrogen and references about it are also covered in the corresponding articles on the summary, introduction, and observation articles on sustainable plant nutrient management.
Sustainable Plant Nutrient Management (SPNM): An overview
Sustainable Nutrient management: Introduction to concept, strategies, and principles
Nutrient conservation and cycling
Mineral fertilizers (including ash) and sustainability
Nitrogen (N): Essential for plant growth and yield but can have high costs to farmers, the environment, and health
Biological Nitrogen Fixation and seeding Legumes for Soil Fertility
The importance and management of Phosphorus (P) and Potassium (K) in plant production
Ion charges and secondary (=meso) nutrients: Calcium, Magnesium and Sulphur
How important are the Micronutrients for plants
Soil and plant analysis and field observations
When are approaches to Plant Nutrient Management actually Sustainable?









































































