How Ozone is used in Food and Beverage Industry
Ozone: Safety and Sustainability in Food and Beverage
Ozone has gained greater popularity recently as a result of rising consumer demand for “greener” food additives, regulatory approval, and increasing consensus that ozone is an environmentally advantageous technology. The powerful oxidant ozone efficiently eliminates many bacteria that can be encountered on fruits, vegetables, grains, meat, and their products. The ability of ozone to perform a variety of functions makes it an intriguing food processing agent.

History of Ozone
A Dutch chemist called Van Marum was probably the first to detect ozone gas sensorially. In the description of his experiments, he mentioned the notion of a characteristic smell around his electrifier. He highlighted the idea of a distinctive smell around his electrifier when describing his experiments. The discovery of ozone, however, wasn’t even referenced by name until decades later, in a Schönbein essay from 1840. Schönbein had noticed the same characteristic smell during his experiments that Van Marum had tried to identify earlier (Picture 1). He gave this gas the name “ozone,” which is distracted from ozein; the Greek word for scent. Typically, Schönbein is acknowledged for discovering ozone. Additionally, Schönbein is recognised as being the first to study how ozone and organic materials react.

Picture 1. Van Marum`s Frictional Electrostatic Machine 1785
Von Siemens created the first ozone generator in Berlin. This manufacturer also published a book on using ozone in water. This led to a number of trial projects, during which the ozone’s disinfectant mechanism was studied (1).
Ozone was introduced for the first time on a large scale technically in Oudshoorn, Netherlands, in 1893. This ozone installation was also thoroughly studied by French scientists. The first major ozone installations went into operation in Nice in 1906 and in St. Petersburg in 1910 (2). Since then, ozone has been applied in Nice continuously, causing Nice to be called the ‘place of birth of ozone for drinking water treatment’. The use of ozone installations increased in a number of nations in the years before World War I. Nevertheless, this growth quickly stopped. This was related to the study of poisonous gases, which undoubtedly contributed to the creation of chlorine. This disinfectant seemed like a good substitute for ozone because it didn’t have the management issues that ozone does, such as low applicative guarantee and low ozone-generating yield. The total number of operational ozone installations around the world had only increased to 119 by 1940. This number had grown to 1043 ozone installations by 1977. France was home to more than half of the installations. Over 2000 applied ozone installations were thought to have been installed around 1985.
For water disinfection, chlorine continues to be preferred over ozone in current times. However, over the past decade, ozone application has begun to rise once more. Trihalomethanes (THM), a hazardous disinfection by-product of chlorine treatment, was identified as the cause of this in 1973. As a result, scientists began looking for substitute disinfectants. An increase in irritating, challenging-to-remove organic micropollutants in surface waters was another issue. Ozone appeared to oxidize these substances more quickly than chlorine and chlorine compounds. Ozone has also been shown to destroy microorganisms like Cryptosporidium that become resistant to disinfectants. Finally, there has been improvement in the elimination of ozone management issues.
Microbial Inactivation by Ozone
Ozone kills bacteria by gradually oxidising vital cellular components. Ozone’s strong oxidising impact, which permanently damages biological macromolecules including proteins and DNA as well as the fatty acids in cell membranes, is the foundation of its antibacterial activity (3, 4). The main method for inactivating bacteria is direct interaction with molecular ozone (5) (Picture 2), while some kinds of bacteria are rendered inactive by indirect interactions with radicals (6). There is no evidence of ozone disinfection resistance due to the mechanism of ozone action, which eliminates the bacteria through cell lysis (7).
Generation of Ozone
By splitting the oxygen (O2) molecule in the air into free radical oxygen, ozone (O3) is created. Ozone is created when a single oxygen (O) molecule joins with the available oxygen (O2) to make ozone.
O2 + hv⟶2O
O2 + O⟶ O3
O3 ⟶ O− + O2
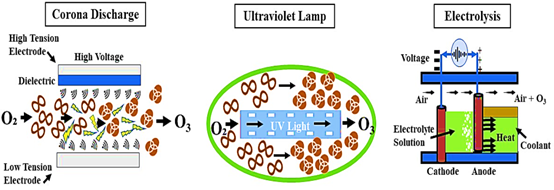
The sun’s UV radiation and lightning discharge are the sources of this enormous energy in nature. Ozone is unstable and breaks down once more into an oxygen molecule. Ozone can be produced on-site as needed using a variety of methods, three of which are currently commercially available – corona discharge, UV radiation and electrolysis (Picture 3).
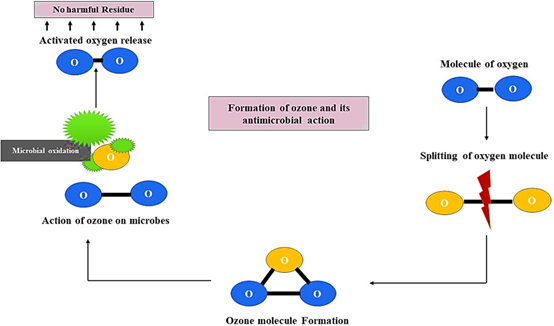
Picture 2. An illustration of how ozone is generated and how it destroys bacteria (8)
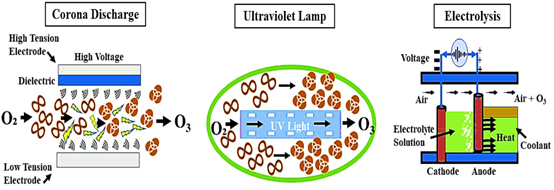
Picture 3. An illustration of the various techniques used to generate ozone (8)
Ozone Applications in the Food Industry
Before ozone was classified as GRAS in 1995, it was used only for disinfection of drinking water (9, 10, 11). In June 2001, ozone, in gas and aqueous phases, was approved by the US Food and Drug Administration (FDA) as an antimicrobial additive for direct contact with foods (12). Nowadays, ozone is a widely used commercial technology in the food industry for a variety of purposes, including:
- irrigation and soil treatment (13),
- avoiding the use of toxic chemicals when spraying crops (14),
- odour control in animal housing (15),
- uses in food processing plants for water and air treatment (16),
- food decontamination and disinfection (12),
- controlling microbial contamination (Cleaning-in-place, biofilms) in the food industry (17),
- safe packaging and storage (18)
- stop ripening and spoilage (19).
Ethylene is totally oxidised by ozone, leaving just carbon dioxide, water, and oxygen. Following is the reaction.
C2H4 + 6O3 à 2CO2 + 2H2O + O2
Ozone is considered to be one of the promising disinfection tools of the future thanks to its multifunctional properties (16). Ozone can be applied in a gaseous or liquid form, and it is particularly effective at eliminating bacteria because it is poisonous to them even at quantities as low as 0.01 ppm.
Regarding the sanitation objective, chlorine and other non-oxidative biocides were frequently employed to reduce microorganisms by a 2-log unit. While ozone induces extensive oxidation of interior cellular proteins, which causes fast cell death, chlorine, the most widely used disinfectant, selectively destroys certain intracellular enzyme systems (20). However, viruses are resistant to chlorine. Since the process for microbial reduction involves penetration through the membrane, it also requires a higher concentration and longer exposure time than ozone. Since ozone is an oxidative biocide, it is preferable to non-oxidative ones for preventing unpleasant tastes or cancer-causing effects. Ozone is 3000 times more powerful than chlorine. The use of ozone for sanitation of surfaces and equipment has shown outstanding results in terms of microbe control and cost savings due to less chemical handling and maintenance in the beverage manufacturing sector (21) (Picture 4). Positive results have been observed in red meat processing plants also (22).
Picture 4. Luminometer tests of the total biomass reduction on the production tables of a meat snack company after overnight gaseous ozone treatment. A thorough mechanical & chemical cleaning prior to disinfection with gaseous ozone is necessary (photo from personal archive).
Advantages and drawbacks of Ozonation
Listed are the advantages and drawbacks of the ozone disinfection techniques suggested for applications involving fresh-cut organic fruits, vegetables, and meat products (19).
Advantages of ozone treatment
- High antimicrobial activity in terms of concentration and duration as compared to nonoxidative biocins (chlorine).
- Less contact time is required for disinfection compared to other disinfection techniques.
- There is no residual issue because it is fully utilised and becomes reduced.
- No need to store hazardous materials in comparison to other sanitation methods.
- Non-toxic at low ppm (less than 4 ppm) and effective in bactericidal usage.
- Lower running costs; only oxygen cylinder filling and power supply expenses are important.
- No heat generation or requirement (applies to heat-sensitive meals), which reduces the need for input energy.
- Reduces the expense of storing petrol and transporting cleaning chemicals; an environmentally and economically viable technology.
Drawbacks of ozone treatment
- Ozone is toxic; when inhaled it causes throat and nasal problems, and even leads to asthma.
- Ozone is a highly unstable gas, so controlled release on requirement needs to be established.
- Recontamination problems in CIP pipes, as ozone decomposes completely within a short duration.
- Corrosive at high ppm (higher than 4 ppm), care should be taken in using ozone and releasing it to the treatment chamber.
- It requires regular monitoring in indoor applications for any leakages.
- Higher initial investment for the generation equipment.
- Because it is unstable and not appropriate for storage, generation on-site is necessary.
In conclusion, ozonation is a cutting-edge technology that helps preserve an uncontaminated food supply chain without damaging the environment. Ozone treatment ensures that food’s physicochemical, nutritional, and sensory qualities are retained (19). The FDA and FSIS/USDA’s regulatory approval of ozone use in food removes all regulatory barriers to its industrial implementation. To use ozone effectively and safely, treatment conditions should be properly established for all products and materials.
References
- https://www.lenntech.com/library/ozone/history/ozone-history.htm
- Kogelschatz U, “Advanced Ozone Generation” in: Process Technologies for Water Treatment S. Stucki Ed. (Plenum Press, New York,1988) pp.87-120
- Hoffman, R.K. (1971) Toxic gases. In Inhibition and Destruction of the Microbial Cell ed. Hugo, W.B. pp. 225–258. London: Academic Press.
- Naitoh, S. (1994) Inhibition of food spoilage fungi by application of ozone. Japan J Food Micro-biol 11, 11–17
- Finch, G.R., Yuen, W.C., Uibel, B.J. (1992) Inactivation of Escherichia coli using ozone and ozone hydrogen peroxide. Environmental Technology 13:571-578.
- Bancroft, K., Chrostowski, P., Wright, L., Suffet, I.H. (1984) Ozonation and oxidation competition values: Relationship to disinfection and microorganism regrowth. Water Research, 18(4):473-478 https://doi.org/10.1016/0043-1354(84)90156-8
- Pascual A., Llorca I., Canut A. (2007) Use of ozone in food industries for reducing the environmental impact of cleaning and disinfection activities. Trends Food Sci. Technol., 18:29–35. https://doi.org/10.1016/j.tifs.2006.07.015
- Dubey, P., Singh, A., Yousuf, O. (2022) Ozonation: An Evolving Disinfectant Technology for the Food Industry. Food and Bioprocess Technology, 15:2102–2113, https://doi.org/10.1007/s11947-022-02876-3
- Von Gunten, U. (2003) Ozonation of drinking water: part I. Oxidation kinetics and product formation. Review, Water Res, 37(7):1443-67. https://doi.org/10.1016/S0043-1354(02)00457-8
- Von Gunten, U. (2003) Ozonation of drinking water: part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Review, Water Res, 37(7):1469-87. https://doi.org/10.1016/S0043-1354(02)00458-X
- Chand, R., Bremner, D.H., Namkung, K.C., Collier, P.J., Gogate, P.R. (2007) Water disinfection using the novel approach of ozone and a liquid whistle reactor. Biochemical Engineering Journal, 35(3): 357-364. https://doi.org/10.1016/j.bej.2007.01.032
- Sarron, E., Gadonna-Widehem, P., Aussenac, T. (2021) Ozone Treatments for Preserving Fresh Vegetables Quality: A Critical Review. Foods, 10, 605. https://doi.org/10.3390/foods10030605
- Martínez, S.B., Pérez-Parra, J. & Suay, R. (2011) Use of Ozone in Wastewater Treatment to Produce Water Suitable for Irrigation. Water Resources Management, 25, 2109–2124. https://doi.org/10.1007/s11269-011-9798-x
- Steffen, H. & Rice, R.G. (2008) The PhytO3 Tech Crop Protection Technology for Microorganism and Insect Control using Ozone, UV, and Dipole-Electrical Air Jet Spray Technologies – Technical Basis and Possible Chemistries Involved, Ozone: Science & Engineering, 30:3, 216-227, https://doi.org/10.1080/01919510701864270
- Alkoaik Fahad (2009) Ozone Treatment of Animal Manure for Odor Control. American Journal of Environmental Sciences, 5(6), https://doi.org/10.3844/ajessp.2009.765.771
- Guzel‐Seydim, Z., Greene, A., Seydim, A.C. (2004) Use of ozone in the food industry. Lwt – Food Science and Technolog, https://doi.org/10.1016/J.LWT.2003.10.014
- Panebianco, F., Rubiola, S., Di Ciccio, P.A. (2022) The Use of Ozone as an Eco-Friendly Strategy against Microbial Biofilm in Dairy Manufacturing Plants: A Review. Microorganisms, 10, 162. https://doi.org/10.3390/microorganisms10010162
- Smilanick, J.L. (2003) Use of Ozone in Storage and Packing Facilities. Washington Tree Fruit Postharvest Conference. http://postharvest.tfrec.wsu.edu/PC2003H.pdf
- Prabha, V., Barma, R.D., Singh, R., Madan, A. (2015) Ozone Technology in Food Processing: A Review, Trends in Biosciences 8(16), Print: ISSN 0974-8, 4031-4047.
- Fetner, R.H., Ingols, R.S (1959) Bactericidal activity of ozone and chlorine against Escherichia coli at 1 °C. In: Ozone Chemistry and Technology, Adv Chem Series No 21. Am Chem Soc, Washington, p. 370-374.
- Williams, R.C., Sumner, S.S., Golden, D.A. (2004) Survival of Escherichia coli O157:H7 and Salmonella in apple cider and orange juice as affected by ozone and treatment temperature. J Food Prot, 67(11):2381-6, https://doi.org/10.4315/0362-028x-67.11.2381
- Botta C, Ferrocino I, Pessione A, Cocolin L, Rantsiou K. (2020) Spatiotemporal distribution of the environmental microbiota in food processing plants as impacted by cleaning and sanitizing procedures: the case of slaughterhouses and gaseous ozone. Appl Environ Microbiol 86:e01861-20. https://doi.org/10.1128/AEM.01861-20










































































