How important are the Micronutrients for plants
Micronutrients: No problem, hidden hunger or visibly damaged on young plant parts and consumers?
Micronutrients can be difficult for plants to take up in special soil conditions or for some crops – and sometimes be taken up in large-toxic quantities. Deficiency symptoms occur in the young parts, fruits, seeds, or root nodules of nitrogen-fixing legumes. However, early detection can be hard, and deficiency of one nutrient can block plants from using another. See the Cover picture and Figures 1, and 2.
The ions can be more or less oxidized, affecting availability and functions as enzymes, etc. The micronutrients are needed in very low concentrations in plants (part per million, ppm) in enzymes that promote processes (serving as catalysts). The opposite of oxidation is reduction (of electric charge).
Several micronutrients can be important, but locally a few are essential for some combinations of soils and crops: Often Manganese (Mn, not Mg), Iron (Fe, Fe2+, Fe3+), Zinc (Zn, normally Zn2+), Copper (Cu, Cu+, Cu2+) or Boron (B). Fe and Zn deficiencies for people are common in at least low and medium-income countries (as well as, e.g., vitamin A, but vitamins are only micronutrients for people and animals).
Within normal pH ranges, the availability of micronutrients all falls with increasing pH except for Molybdenum (Mo), which increases[1]. Mo occurs in soils as negatively charged Molybdate anions[2]. For Boron, high and low pH can be a problem. [3] Liming tropical soils above pH 5.5 is no longer recommended. At lower and more acidic pH, Aluminium (Al3+ is the toxic form) and Manganese (Mn) toxicity can often damage the root. Aeration (drainage) of the soil matters, too, because the micronutrients can be more or less oxidized and available. Excess of some can also be a problem in some situations.
Crop micronutrient deficiencies are associated in complex ways with soil pH, form and type of organic matter, texture, and soil water as observed for major crops in Michigan, Indiana, and Ohio in the central USA[4].:
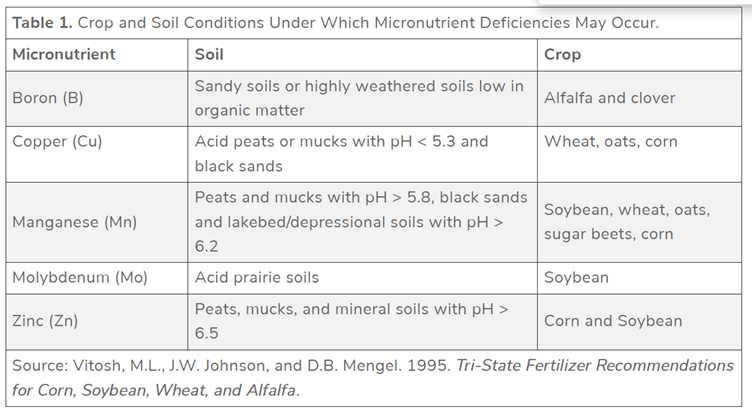
The same source gives access to corresponding tables with e.g. adequate nutrient concentration in parts per million (ppm) with relevant sampling methods
Severe symptoms can be observed (first) on young leaves. More specifically, in legumes, molybdenum deficiency damages nitrogen fixation, and contrary to other micronutrient deficiencies, it is most common in soils with low pH. Boron deficiency can, e.g., be one cause of poor seed formation on the tip of maize cobs.
Direct testing for Molybdenum (Mo) is expensive, but narrow “whiptail-” or cup-shaped leaves of broccoli and cauliflower is an indication of deficiency as well seeds germination prematurely, e.g., at maize cobs. Other symptoms are caused by the need for Mo to fix or use nitrogen by bacteria and plants.
Fertile organic matter can help with a steady supply of micronutrients, and only some mineral fertilizers contain some of the micronutrients, and they can cost more. Boron (B) deficiency is most common in soils low in organic matter and occurs in many parts of the world. However, organic soils (peats or mugs) are those most likely to be deficient in available Copper (Cu) if they are acidic, and else Manganese (Mn) and Zinc (Zn). While acidity makes micronutrients more available in soils (except for molybdenum), it also makes them more available to be leached out and deficient, e.g., in the humid tropics.

Figure 1. Copper (Cu) deficiency in e.g. maize starts at the younger parts with yellowing and sometimes spiralling. Permission: Maize – Crop Scout Pocket Guide. African Plant Nutrition Institute, APNI.net.
Micronutrients can vary in charge with oxidation level
Micronutrients are relatively complex, and this article is only an introduction to which may be most relevant for a crop and combination. They are usually in the middle groups of the periodic table of elements, and their availability depends on how oxidized they are. While the first main group of elements simply and easily gives off one electron to become monovalent, positive cations (K+, and, e.g., Na+), the second group releases two (Ca+2, Mg2+). Instead, micronutrients are transition metals or non-metals that can vary in charge (oxidation levels), but Zinc is normally found as Zn+2. See relevant versions of periodic tables via reference[5].
Accordingly, flooding of wet rice (paddy) soil changes the availability of micronutrients:
Flooding can increase the availability of iron (Fe) and manganese (Mn). However, at the same time, flooding reduces the availability of Zinc (Zn) and Copper (Cu). Field capacity corresponds to the soil about 70 cm higher than water-saturated soil, resulting in about as much water and air in the pores (ideal). Farmyard manure improves grain concentration of iron for any water condition. Mn concentration in grains was not controlled by soil Mn availability. Interaction among ions affects uptake (e.g., competition of ions with similar charge).

Figure 2. Boron (B) deficiency in e.g. maize starting at the younger parts with twisting; yellow or white spots can occur plus lack of seed formation in the tip of cobs. Permission: Maize – Crop Scout Pocket Guide. African Plant Nutrition Institute, APNI.net.
Availability and uptake depend on many factors
Soil nutrient availability can depend on pH, organic matter changes, fertilizers, microbiology, and, as mentioned, aeration (reduction-oxidation capacity = ‘redox potential’)[6]. Small organic acid molecules increase the availability of Cu by dissolving organic acids, but bigger, dark molecules like humic acid decrease it by binding Cu. Accordingly, deficiency can be found in acid-organic soils like peats, mugs, and black sands.
Uptake capacity also depends on the plant’s excretions, root hair length, and root-associated fungi and bacteria – plus roots ’capacity as a sink removing dissolved nutrients so new dissolve and are taken up. These capacities vary both between and within species. Tolerance to other soil problems, like acidity, also helps effective root functions.
Other elements may benefit some plants or other organisms but are rarely or never deficient in field-grown plants. These include Chlorine (Cl, needed by some palms and often in excess), Sodium (Na), Cobalt (Co), needed in minute amounts for nitrogen fixation, Iodine (I), and Nickel (Ni). Many other elements are found in plants (apparently) without any function for the plants, animals, and people eating them.
Zinc deficiency is common for people in many types of countries, climates, and soil pH, particularly low and middle-income countries, incl. but not only DRC Congo, from India to Poland, Southeast Asia, Central America, etc. For free maps and details, see Wessel and Brown (2012), who found low food consumption – particularly of animal products- was an indicator, and 17% of the world’s population was at risk of Zn deficiency [7]. Fertilizer use can be a part of the problem (if it does not contain Zn) or of the solution it does (e.g., a small quantity of harmless ZnSO4). Likewise, improved crop types can harm (with less diverse food if few crop species still are improved) or help when selected for zinc uptake and availability (e.g., beans and rice).
References:
[1] (PDF) Soil pH Affects Nutrient Availability (researchgate.net)
[2] Molybdenum – NSW | Fact Sheets | soilquality.org.au
[3] Boron reactions in soils (borax.com)
[4] Corn, Soybean, and Alfalfa Yield Responses to Micronutrient Fertilization in Ohio | Ohio line (osu.edu)
[5] Oxidation states table of the elements (chemix-chemistry-software.com)
[6] Masunaga T, Fong J D M (2018): Chapter 11 – Strategies for Increasing Micronutrient Availability in Soil for Plant Uptake, Editor(s): Hossain M A, Kamiya T, Burritt D J, Tran L-S P, Fujiwara T: Plant Micronutrient Use Efficiency, Academic Press, 195-208. https://doi.org/10.1016/B978-0-12-812104-7.00013-7.
[7] Wessells KR, Brown KHE (2012): Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7(11):e50568. doi: 10.1371/journal.pone.0050568. Epub 2012 Nov 29. PMID: 23209782; PMCID: PMC3510072.
Reetz H F (2016): Fertilizers and their Efficient Use. IFA, Paris, France, May 2016 Library (fertilizer.org)
Sustainable Plant Nutrient Management (SPNM): An overview
Sustainable Nutrient management: Introduction to concept, strategies, and principles
Nutrient conservation and cycling
Mineral fertilizers (including ash) and sustainability
Biological Nitrogen Fixation and seeding Legumes for Soil Fertility
The importance and management of Phosphorus (P) and Potassium (K) in plant production
Ion charges and secondary (=meso) nutrients: Calcium, Magnesium and Sulphur
How important are the Micronutrients for plants
Soil and plant analysis and field observations
When are approaches to Plant Nutrient Management actually Sustainable?









































































