E.U. Food Supplements Labelling

This post is also available in:
This post is also available in:
![]() Français (French)
Français (French)
What you need to know about those pills/powders/drops you use to boost your health
What is a food supplement?
According to the definition laid out in E.C. Directive 46/2002, food supplements are “foodstuffs the purpose of which is to supplement the normal diet and which are concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose form, namely forms such as capsules, pastilles, tablets, pills, and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles, and other similar forms of liquids and powders designed to be taken in measured small unit quantities”.
In this definition, what is important, what we need to focus on, is the “form” in which food supplements are marketed. When in doubt if you’re buying a food supplement or not, take a look at its form.
Just to make it clearer, a “regular food” which is vitamin or mineral added, is NOT a food supplement. Think about those “meal-replacing bars” that can be found in the “dietary” section of each store.
Even if it may seem that those products fall into the food supplements category, they don’t. And that’s mainly because of their composition but, most of all, because of the form they are marketed.
What information must be printed on a food supplement label?
According to E.C. Directive 46/2002, the term “food supplement” must be printed on the label. This means that food supplements are “standardized” food; if a particular food falls into the above definition, it must be named: “food supplement” and no other name can be used.
Then, food supplements follow the same rules as all other food, when speaking of labeling. This means that E.U. Regulation 1169/11 applies as a whole.
Mandatory information that must appear on a food supplement label are:
- Product name (the above-mentioned “food supplement”);
- Net weight, expressed in mass for solids and volume for liquids.
- Name and address of an E.U.-established food business operator;
- List of ingredients (all ingredients that make up the food supplement, preceded by the word “ingredients”, listed in descending order of weight);
- The quantity of certain ingredients (% labeling), when those ingredients are highlighted on the label with words or images;
- Allergens (clearly listed in the ingredient list with a font/color such as to make them distinguishable from other ingredients and allow their immediate identification);
- Best before date or use by date (the latter is used for those food supplements that, after a short period, may be dangerous for consumption);
- Storage conditions (typically, a statement like “store in a cool and dry place, away from direct sunlight” or any other indication that may allow the correct storage of the product);
- Country of origin, if needed (this indication is often mandatory when its omission could mislead the consumer about the actual origin of the food);
- Instruction for use, in case their omission may make difficult the correct consumption of the food supplement (this typically applies to those supplements that require specific instructions for their preparation).
- Product name and net weight must be written in the same field of vision

In addition to those, E.C. Directive 46/2002 gives us a bit more to be concerned about, when designing our food labels. The following information must be added to each food supplement label:
- the names of the categories of nutrients or substances that characterize the product or an indication of the nature of those nutrients or substances;
- the portion of the product recommended for daily consumption (that is determined by the manufacturer to provide the claimed nutritional, physiological effect);
- a warning not to exceed the stated recommended daily dose;
- a statement to the effect that food supplements should not be used as a substitute for a varied diet;
- a statement to the effect that the products should be stored out of the reach of young children.
The nutrition information for food supplements follows particular rules, that are slightly different from those of standard food.
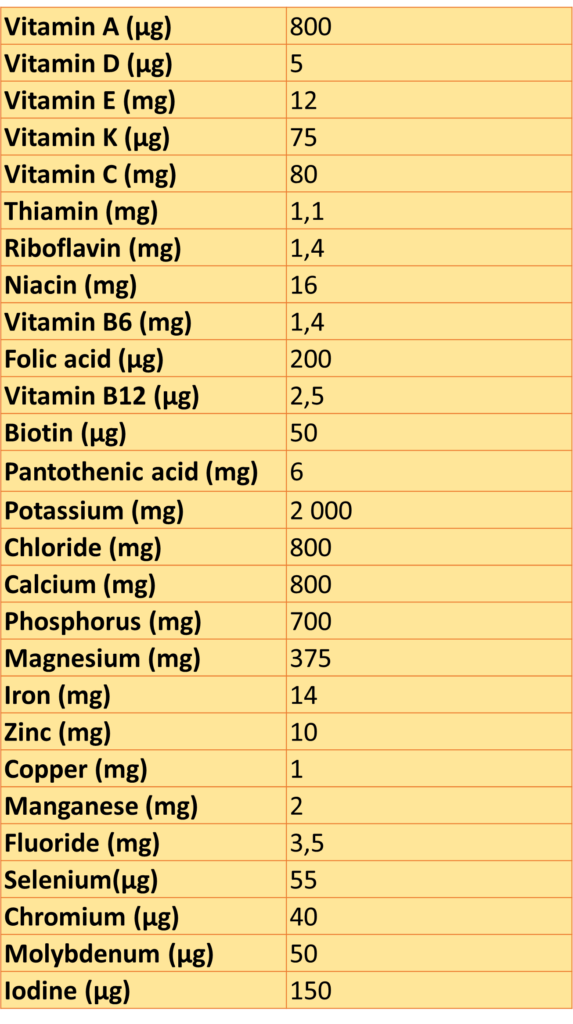
The only required nutrients to be listed are those effectively used in the product (vitamins, minerals, or other substances). These are going to be listed “per portion”, according to the portion recommended for daily consumption and, in addition to that, as a percentage of the reference values set in Annex XIII Part A of E.U. Regulation 1169/11:
DAILY REFERENCE INTAKES FOR VITAMINS AND MINERALS
Particular case: botanicals
One particular category of ingredients that can be added to food supplements is the so-called “botanicals”, otherwise known as plants or parts of them that can be used as an ingredient. These ingredients are subject to E.U. Regulation 2283/2015 on novel foods. This means that each ingredient must be evaluated based on its “common use” in food. If the use is consolidated before 1997, then no action is required. Otherwise, the ingredient must be notified to E.U. Commission, that will eventually approve its use.
For a complete list of novel food, it is possible to consult the E.U. database at https://webgate.ec.europa.eu/fip/novel_food_catalogue/
Here is where the good news end. The above-mentioned catalog tells you only if a given substance is allowed or not, but does not tell you anything about eventual limits in its use or warnings to be printed on a label.
This is because, at the E.U. level, no harmonized Regulation exists. Therefore, each Member State is free to adopt its own National Law.
Some minor efforts have been made in the last years, with the aim to uniform the list of botanicals across Europe. The most “ambitious” project thus far, is the BELFRIT list, which unifies the lists of Belgium, France, and Italy. The goal of the BELFRIT project is to obtain a common list of eligible plants for use in food supplements, comparing the three national lists of plants allowed. The plants have been evaluated on the basis of the traditional use and of the whole complex of available scientific evidence. After a year and half of work the three Countries arrived at the definition of a common list of plants whose conventional preparations are allowed to use.
The BELFRIT project is a guide to direct the manager and the operator decisions. It provides a precise identification of plants that may be used in the manufacturing of food supplements. It also indicates some key points to control in the production, starting with the nature of the chemical constituents that are present in the species and which should be monitored.
Particular case: other substances with physiologic or nutritive effect
This category encompasses all those other substances which are not vitamins, minerals, or botanicals. The list may include, for example, amino acids, flavonoids, enzymes, melatonin, etc.
As per the botanicals above, no harmonized Regulation exists at E.U. level. And just like for botanicals, the same regulatory framework applies, meaning that each substance must be tested against the novel food catalog in the first place. Then, each Country’s Regulation kicks in, regarding the allowed limits or warnings.
References
- DIRECTIVE (EC) No 46/2002 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements
- REGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 25 October 2011 on the provision of food information to consumers
- REGULATION (EC) No 1170/2009 of 30 November 2009 amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards the lists of vitamin and minerals and their forms that can be added to foods, including food supplements